Research Themes in the Translational Medicine Living Lab
The research themes developed by the partners of the Translational Medicine Living Lab include several applications of advanced biomaterials mainly in the healthcare field. The regerative orthopaedics and neurology have been the focus of the research activities developed within the RINOVATIS project, aiming at solving pathologies related to the Central Nervous System (CNS) and the Perypheral Nervous System (PNS) as well as regenerating bone and cartilage tissues affected by Osteochondral (OC) defects.
Silver based antibacterial treatments
The growing resistance to conventional antibiotics and the great concerns about the healthcare costs have recently encouraged the development of novel approaches in the prevention of infections. The antimicrobial properties of silver against a broad spectrum of bacteria, fungi and viruses are well known since a long time and silver is used today as antimicrobial agent in a wide range of applications. The deposition of silver nano-coatings on natural and synthetic substrates can open new horizons towards the development of advanced antibacterial biomaterials.
At the Department of Engineering for Innovation (DII) of Università del Salento, a silver deposition technology has been developed and applied to many materials for different applications. The process, based on the in situ photochemical synthesis and deposition of silver coatings, involves the impregnation of the surface with a silver-based solution and the UV irradiation of the wet substrate. The perfect adhesion of the silver coating to the substrate, the long-term antimicrobial capability and the good biocompatibility are some of the most important results obtained.
Silver treated wound dressings have been designed for prevention of infections associated to critical wounds, burn and diabetic ulcers; smart textile materials with antimicrobial properties have been obtained for both biomedical application and daily comfort.
Silver coatings have been also developed for prevention of biofilm growth and infections on implantable medical devices such as catheters, prosthesis and scaffolds for bone and wound regeneration.
Cartilage Scaffolds
Porous scaffold realized in collagen and chitosan for regenerating cartilage could be obtained through the freeze-drying technique by the Department of Engineering for Innovation (DII) of Università del Salento, in collaboration with Ospedale San Raffaele, CNR-NANO and Università di Bari (Plasmalab). Collagen scaffolds show a regular and homogeneous structure with an isotrope porosity, with pore size of 0.11 mm in both longitudinal and transverse directions.
Subsequent reticulation of the material allows the significant increase of mechanical resistance. It is also possible to seed the scaffold with chondrocytes in order to obtain a system with properties similar to those of the cartilage, employing fibrin glue to promote chondrocyte survival in the type I collagene. Fibrin glue also contributes to sustain the synthetic activity of chondrocytes within the scaffolds, making the latter biocompatible and mechanically, morphologically and chemically suitable for cartilage tissue regeneration.
-
The image represents a collagen scaffold for cartilage regeneration.
Bone Tissue Scaffolds
Among the different ceramic materials, calcium phosphate raise great interest as bone tissue substitutes thank to their biocompatibility and bioactivity, aspects that make them specifically suitable for their use in the orthopedic and dental technology sectors. From the crystallographic point of view, hydrossiapatite (HA) is the most similar to the inorganic phase of the bone tissue, thus representing the best structural substrate for bone tissue growth. The Department of Engineering for Innovation (DII) of Università del Salento, in collaboration with Ospedale San Raffaele and CNR-NANO designed innovative methods for synthetizing hydrossiapatite, analyzing their effects on the chemical and morphological properties of the HA powder. Polymeric (collagen) and ceramic (HA) materials could also be combined to obtain a scaffold able to simultaneously regenerate cartilage and bone tissue for a complete osteochondral defect treatment. Innovative techniques of 3D scaffold fabrication via plasma technologies have also been developed, supported by the partner Università di Bari (Plasmalab), employing porous HA and polymeric materials that improve their biocompatibility and their mechanical, morphological and chemical suitability for the regeneration of bone tissue.
-
The image representa a bi-layer scaffold, made of collagen (top layer) and HA (bottom layer).
PNS Scaffolds
The devices have been designed by the Department of Engineering for Innovation (DII) of Università del Salento, in collaboration with Ospedale San Raffaele, CNR-NANO and Università di Bari (Plasmalab) aiming at obtaining high mechanical resistance, biodurability (to resist enzymatic digestion) and biocompatibility. The chemical-structural, thermos-physical and morphological properties of 2 industrial sources of type I collagen, both FDA approved (one isolated from bovine derm and the other from equine tendon) have been characterized by means of electrophoresis (SDS-PAGE), infrared spectroscopy (FT-IR), calorimetry (DSC), thermogravimetric analysis (TGA) and microscopic techniques (SEM). The synthetic technology is based on the combination of a spin-casting and freezing technique and of a freeze-drying process, which determine sublimation of the freezing obtained crystals and lead to a high porous structure. Post-fabrication reticulation treatments allows controlling and tuning the stiffness and durability of the devices, preserving their biocompatibility and making them suitable for regenerating the nervous tissue and its mechanical, morphological and chemical properties.
-
Different types of scaffolds of different sizes, suitable for 30-mm gap:
-
• Tubular neural guides
-
• Longitudinally porous matrix
Controlled release devices
CNR–NANO
- , in collaboration with
- ,
- and
- studied the best strategies to integrate capsules into different 3D scaffolds in order to realize the biological factors for the regeneration of both PNS and OC tissues. Development and optimization of the protocol for the synthesis of polyelectrolyte-based capsules, through the layer-by-layer (LbL) deposition technique, enabled the production of microcapsules composed by a cavity and a multilayer wall. Both compartments could be functionalized with bioactive molecules.
- Poli(lactic-co-glicolic) acid (PLGA) microspheres could also be synthetized, specifically by
- through the water/oil/water (w/o/w) double emulsion technique with subsequent evaporation of the organic solvent. This technique is ideal for loading PLGa with hydro-soluble molecules, such as proteins and other possible molecular regulators that could be gradually released in the target site, either through diffusion into the polymeric matrix composing the wall or exploiting the hydrolytic erosion of the matrix itself. The chitosan could also be employed to realize microsphere for the controlled release of growth factors.
- Rising the concentration of molecular regulators within the scaffolds through the immobilization of a large number of capsules, containing a high dose of regulators, on the outer and inner wall of the scaffold itself
- Allowing the load of different regulators within the scaffold through its functionalization with capsules containing such regulators
- Tuning the release of one or more regulators varying the capsule degradation kinetics
- Integrating polyelectrolyte within 3D porous scaffolds.
Scaffold functionalization
Plasma processes, mainly developed by Università di Bari (Plasmalab), could be employed for inserting nitrogen and oxygenate groups into the matrices that fill the neuronal guides. Such processes aim at decorating the cross-linked collagen chains with hydrophilic functional groups (included amino and carboxylic groups) in order to activate the scaffold surface thus obtaining multifunctional devices. Processes that could be realized include:
-
• Humid immobilization of microcapsules on type I collagen either pure or plasma modified samples
-
• Plasma processes at atmospheric pressure directly on the biological substrate (cells and culture medium).
CNS Scaffolds
Microstructured scaffold could promote and guide the axonal growth in the CNS, as verified in the studies related to PNS regeneration. Such scaffold has been designed by Ospedale San Raffaele and Department of Engineering for Innovation (DII) of Università del Salento and is realized in collagen, consisting in a tubular pipe, with radial porosity obtained through the spinning technique, and two semi-elliptical lobes, with longitudinal or axial porosity and obtained with the technique of uniaxial freezing, to be inserted in the tubular pipe. The pipe is meant to protect the site of implant from external tissues interferences yet allowing cell to permeate from the inside to the outside of the pipe; the two lobes are meant to facilitate the axonal regeneration of the left and right lobe of the spinal cord, providing the axons with adequate physical and chemotactic support.
-
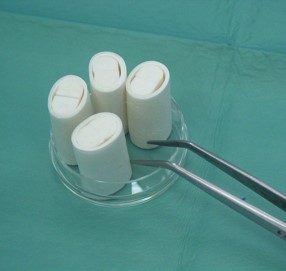
The image represents the collagen CNS scaffolds.
Bioactive Molecules
-
The transcrittomic and bioinformatic analyses enable the investigation, conducted by Innovation Engineering Department (DII) of Università del Salento and Ospedale San Raffaele, about molecules able to promote and guide the regeneration of nervous and osteochondral tissues.
Although the laminin, fibronectin, vitronectin and fibrin create an initial substratefor regenerative processes, their persistence in the peripheral nerve provokes a delay in the regeration process, that ultimately lead to a progressive fiber loss and insufficient axonal regeneration. Potential novel molecules able to promote the regeneration, since they are involved in the organ neurogenesis and morphogenesis, have been studied as well as genes involved in the development of the nervous system have been identified. In particular, genes TMEM158 and EFBN1 appear specifically expressed by the Schwann cells, that compose the axon, and thus represent genes potentially involved in the PNS myelination during the regeneration/development phases.
The astroglial scar in the CNS represents an obstacle to the growth of the axonal stump which is proximal to the damage or rescission. BMP signaling is a biological process that activate astroglial scar formation, but it is possible to encapsulate BMP inhibitors in biodegradable PLGA microspheres.
-
Cartilage and bone matrix production is physiologically regulated by a group of growth factors that acts on their respective cell populations, chondrocytes and osteoblasts, stimulating specific pathways of synthetic activity, ultimately leading to the synthesis and release of matrix protein responsible for the tissue-specific biomechanic properties. IGF-1 plays a fundamental role in promoting cell proliferation and in inhibiting cell death during cartilage regeneration. BMP-2 promotes the recruitment of osteoprogenitor cells and regulates their differentiation towards the osteogenic line while TGFbeta-3 is essential for promoting chondrocyte differentiation.
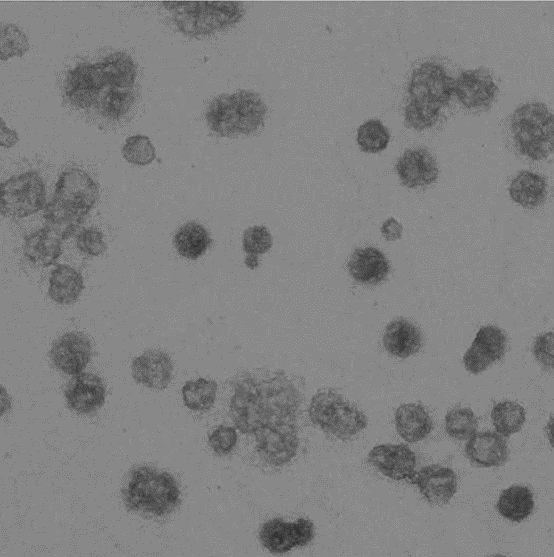
Microvesicles analysis
Cells could communicated and share informations through secretions, inculding extracellular miscovesicles: exosomes and microparticles (MPs) derived from the plasmatic membrane. Exosomes are secreted upon fusion of multivesicle bodies with the plasmatic membrane, whereas MPs originates directly from the plasmatic membrane. Both organelles could control intercellular signal transmission in the encephalon and play a role in the immunemediated demyelination as well as in the neurodegeneration.
Ospedale San Raffaele is able to characterize in vitro the MPs and exosome nature, using myeloid cell culture stimulated with ATP. CNR-NANO has developed a nanoPAS (nanoParticle-Analyzer/Sorter) device for the microfluidic analysis. The nanoPAS is realized via soft-lithography processes and allows separating the nanoparticles through hydrodynamic filtering. The partnership with Engineering Ingegneria Informatica software house enables to realize software algorithms for controlling the nanoPAS microfluidics and for the image processing and analysis of data derived by microvesicles.
-
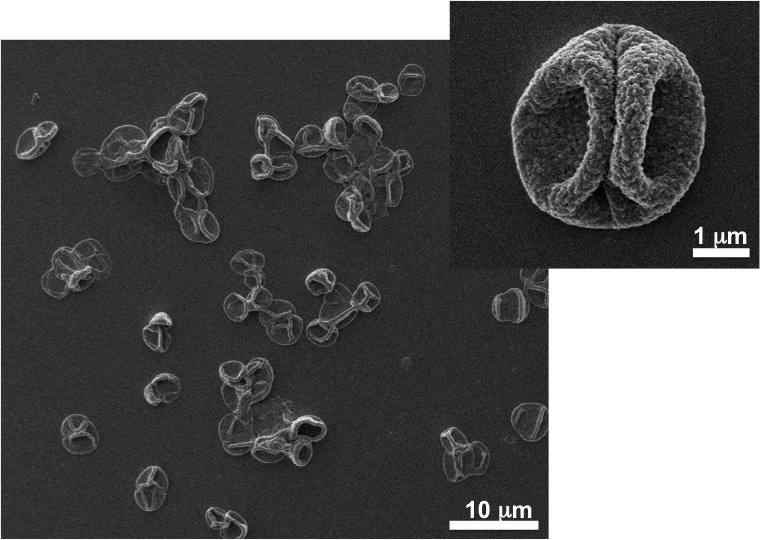
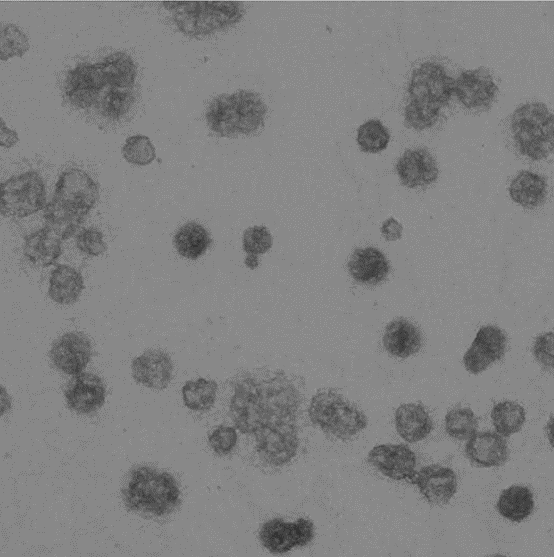
Image represents a microvesicle sample.
Image-based biopsy
The efficacy of in vivo implant of the devices for the regeneration of traumatic lesions in the peripheral nerves could be evaluated by means of novel automatic analysis tools, developed by Ospedale San Raffaele, that allows the automatic analysis of cross-sectional images of nervous tissue obtained by electronic microscopy, thank to the collaboration of DiSTeBA of Università del Salento. The numerical procedures, required for the registration of the images acquired through electronic microscopy, have been consolidated and their automation has been optimized in order to enhance the productivity of the method yet keeping the accuracy of the analysis in the range of that attainable by visual inspection by a trained and skilled clinician.
-
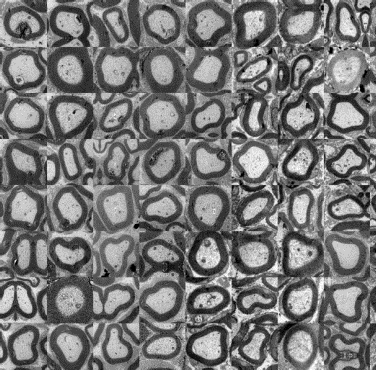
Image represents nervous fibers crossectional slices obtained by means of electronic microscopy as selected, normalizeed and registered through novel visual inspection methodologies developed during the RINOVATIS project.
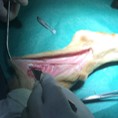
In vivo evaluation
With regards to CNS and PNS traumatic lesions, murine models are to be considered the most valid, due to their cost effectiveness, their low maintenance and the possibility of large cohort studies, their rare post-surgical infections contracted by mice, the availability of validated methods for functional and histopathological analyses. Additionally, ovine models could be employed for the regeneration of long nervous gap. Combining molecular biology techniques, transcriptomic, high resolution imaging, electronic microscopy, transgenesis and neuropathology Ospedale San Raffaele, in collaboration with Department of Engineering for Innovation (DII) and DiSTeBa of Università del Salento is able to assess the relevance of axonal proteic synthesis in physiological (axonal growth, normal adult axon) and pathological (axonal damage) conditions.
Animals commonly used for investigating cartilage tissue regeneration include mice, rabbits, dogs, sheep, horses and pigs. Small sized animals are cost-efficient and low maintenance, although model correlation with expected results on humans could be limited. On the other hand, large sized animals present a cartilage thickness suitable to mimic lesions observed in humans, although being very expensive. Therefore, a multifactorial analysis is required on each animal model when planning in vivo studies. Swine models are mainly used and the degree of cartilage repair/regeneration could be evaluated ex vivo both macroscopically and biomechanically. Additionally, the newly formed tissue could be processed and studied with microscopic, biochemical, immunohistochemical and genic expression methodologies.
-
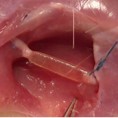
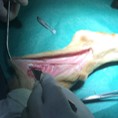
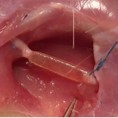
Image represents the in vivo implant of a PNS scaffold in the sural nerve of a sheep.
Process analysis and clinical evaluation
In all the European member states, marketing and clinical use of Medical Devices are regulated by the ddirective 93/42/CEE – Medical Device, and its updates. This norm defines the Minimum Safety Requirements a product should present to be CE marked and, in case of a Medical Device, to be used in the clinical practice, according to the Working Conditions indicated by the maker. Among the main requirements expressed in the Directive, the use-related Risk Analysis and Risk Management are essential. The executing modality of the Risk Management Process, which is part of the Risk Analysis, is defined in the harmonized technical norm EN ISO 14971:2012. Risk quantification and control, risk/benefit evaluation and everything explicitly requested by the norm, are carried out by Dhitech Scarl for the Tissue Engineered Medical Products developed by the partners and for the production of such devices according to the European quality standards. Dhitech Scarl also offers his support in the feasibility analysis of clinical studies in order to verify safety and efficacy of the devices in the clinical practice.